

The CRC Handbook of Chemistry and Physics, 84th edition. There are also many dyes and pharmaceutical agents that contain bromine. Bromine reacts with liquid water to produce hypobromite ion (BrO −), a powerful bleaching agent. Inorganic bromides are important components of photographic emulsions. It is part of group 17 (fluorine family).
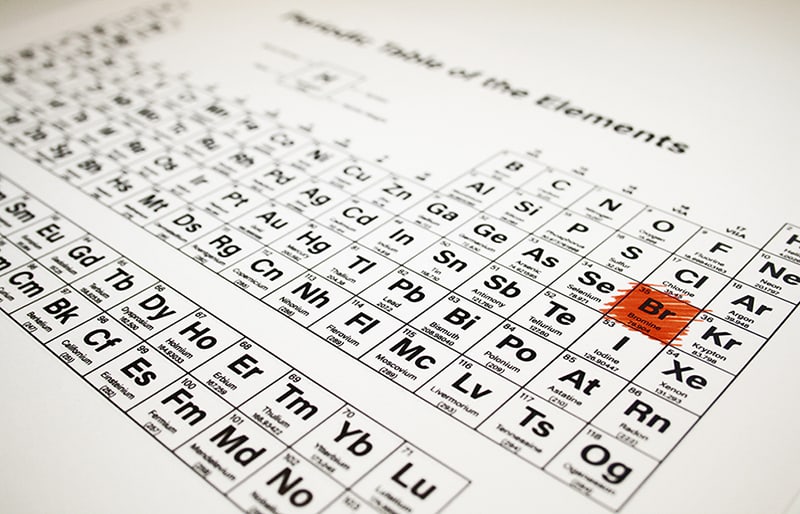
Bromine, symbol Br, has a Base Centered Orthorhombic structure and Red color. Methyl bromide (CH 3Br) is a common agricultural soil fumigant other bromohalocarbon compounds have been used as refrigerants and fire suppressants. Element 35 of Periodic table is Bromine with atomic number 35, atomic weight 79.904. The concentration of bromine in seawater is approximately 67 parts per million (ppm) by weight it is found in Earth's crust at an average level of 3 ppm.īromine compounds have a variety of uses. To learn more about bromine uses, physical and chemical properties, atomic mass and. Bromine forms many interesting covalent compounds as well, including two oxides: bromine (IV) oxide (BrO 2) and bromine (I) oxide (Br 2O).īromine is produced commercially from natural brines and from sea-water either by electrolysis or with displacement by chlorine, a somewhat more reactive halogen. Bromine is a chemical element that has a symbol Br and atomic number 25. These two elements are mercury (Hg) and bromine (Br). Reaction with metallic elements leads to salts such as silver bromide (AgBr), in which the bromine atom has a −1 charge and oxidation number. The periodic table is a chart displaying information about the elements. Bromine will combine with most other elements. Bromine has a pungent, irritating odor that is the source of the element's name (the Greek word bromos means "stench").Įlemental bromine is a diatomic molecule (Br 2). Br Group 17 Period 4 Block p Protons Electrons Neutrons 35 35 45 General Properties Atomic Number 35 Atomic Weight 79. Both the liquid and vapor of bromine are deep red in color. Isolated independently by two chemists, Carl Jacob Lwig and Antoine Jrme Balard, its name was derived from the Ancient Greek, referring to its sharp and pungent smell. Its properties are intermediate between those of chlorine and iodine. It is the third-lightest halogen and is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. As with the other halogens, bromine is very reactive, corrosive, and poisonous. Bromine is a chemical element with the symbol Br and atomic number 35. Balard.īromine is one of two elements (the other being mercury) that is liquid at normal temperatures. Bromine was discovered in 1826 in Montpellier, France, by French chemist Antoine J. Most common ions: Br −, BrO −, BrO 3 −, BrO 4 −īromine is a member of a family of elements known as halogens that are found in group 7A of the Periodic Table.


 0 kommentar(er)
0 kommentar(er)
